This activity is provided by Med Learning Group.
This program is supported by an independent educational grant from Regeneron Pharmaceuticals, Inc.
Copyright © 2025 Med Learning Group. Built by Divigner. All Rights Reserved.
Recognizing and Monitoring for Adverse Events
Recognizing and Monitoring for Adverse Events
Cancer and cancer treatments are known to cause side effects.1 These side effects, also known as adverse events (AEs), can be different for each patient and may vary by medications and treatments.2 Moreover, AEs may be different even among patients who receive the same type of cancer treatment.2
The oncology care team should be aware of the potential for AEs and related symptoms so they can be monitored and prevented or managed appropriately.1-2
Potential Immune-related Adverse Events
Recent advances in immunotherapy have transformed current cancer treatment; however, clinicians must be vigilant for AEs.4-5 As more patients with cancer are exposed to immunotherapy, including immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapies, clinician awareness of the drugs’ associated toxicities and how to best manage them is vital.4-5
While immunotherapy can treat many types of cancer effectively, it can cause the patient’s immune system to attack healthy cells.4 Immune-related AEs (irAEs) can occur at any time during treatment and even after stopping immunotherapy.4 Common irAEs depend on the type of immunotherapy administered.4-5
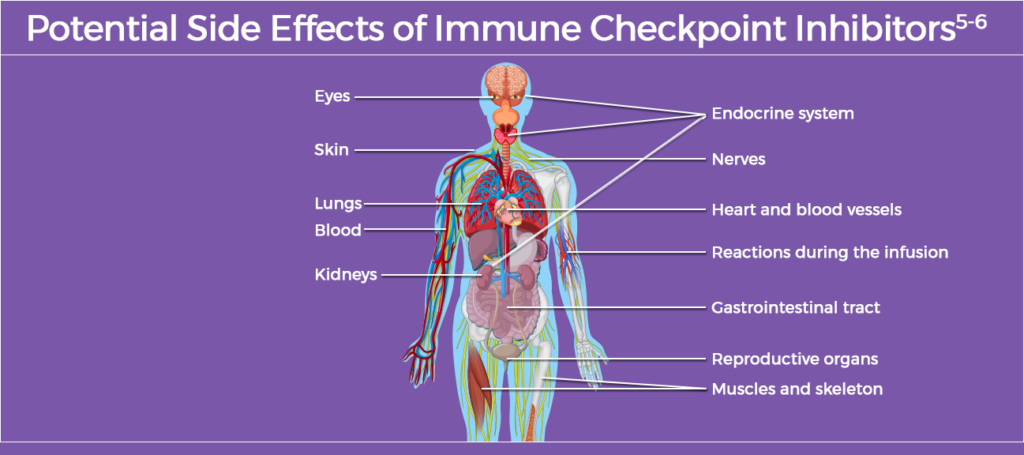
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are the current standard of care for the treatment of several types of cancer, including solid-organ and hematologic malignancies.5 Use of immune checkpoint inhibitors has increased in recent years; in 2023, approximately 56% of patients with cancer in the US were eligible for treatment.6 Development of new immune checkpoint inhibitors continues, making this a rapidly changing field.5
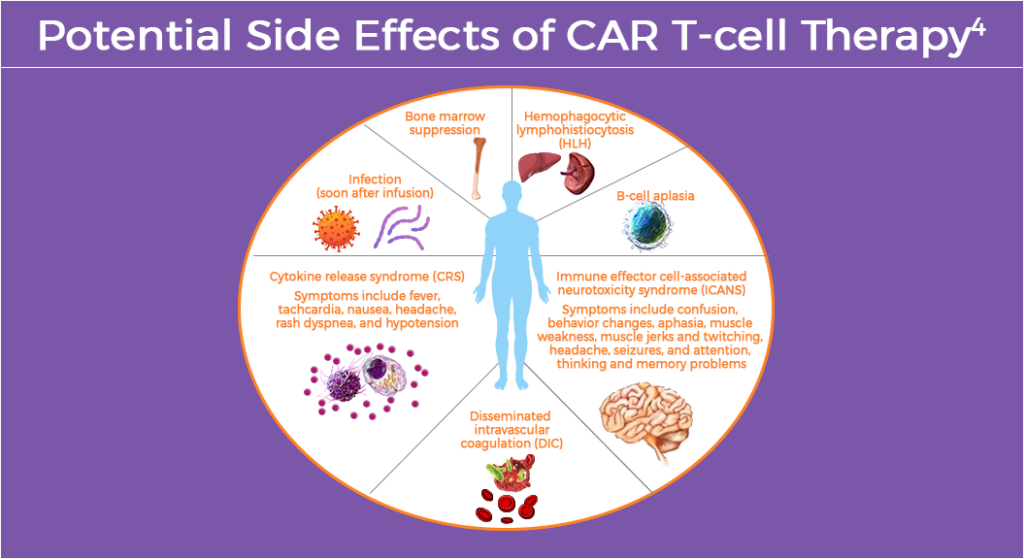
CAR T-cell Therapy
CAR T-cell therapy works by returning adjusted immune cells back into the body to attack cancer cells.4 Common irAEs of CAR T-cell therapy generally result from immune system changes involving more than one part of the body.4
Immunomodulators
Other types of immunotherapy may be associated with AEs.4 Common AEs may include, but are not limited to: cough, respiratory issues, diarrhea, constipation, pain, sweating, headache, confusion, jaundice, seizures, sensitivity to light, weight changes, alopecia or hirsutism, tachycardia, hepatomegaly and splenomegaly.4 While most AEs will resolve or can be treated effectively, some are serious and require immediate medical attention.4
Bispecific Antibodies
Bispecific antibodies have unique toxicity profiles depending on their therapeutic target and format.7 Some require slow dosage escalation and close inpatient monitoring for adverse effects such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome during treatment initiation.7 In addition, on-target off-tumor toxicities may also occur, including B-cell depletion and cytopenias. Other adverse events include infections, cardiovascular complications, venous thromboembolism, and hemophagocytic lymphohistiocytosis.7
Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) which are made up of a monoclonal antibody linked to a cytotoxic payload, increase target selectivity and widen the therapeutic window of traditional chemotherapy.8 However, construct-specific features, such as properties of the payload, drug-to-antibody ratio, and linker stability can contribute to toxicity.8 Clinical manifestations of off-target effects include neutropenia, thrombocytopenia, peripheral neuropathy, interstitial lung disease, hepatotoxicity, ocular toxicity, and other AEs.8,9
Best Practices for Monitoring for Adverse Events
Close monitoring, patient education and supportive care are vital elements of AE monitoring.1-4 Providers should discuss with patients the type of immunotherapy being used, treatment goals, potential AEs, and signs and symptoms to look for.4 The oncology care team can help prevent or treat many AEs related to cancer treatment.4 AEs related to immunotherapy can be mild, moderate or life-threatening, and some effects may not appear until months or years later; treatment depends on severity.4
References
- National Cancer Institute. Side Effects of Cancer Treatment. https://www.cancer.gov/about-cancer/treatment/side-effects
- Centers for Disease Control and Prevention (CDC). Side Effects of Cancer Treatment. Last reviewed: August 26, 2024. https://www.cdc.gov/cancer-survivors/patients/side-effects-of-treatment.html
- American Cancer Society. Managing Cancer-related Side Effects. https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects.html
- American Cancer Society. Immunomodulators and Their Side Effects. Last Revised: December 27, 2019. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/immunomodulators.html
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021;39:4073-4126.
- Prasad V, Haslam A, Olivier T. Updated Estimates of Eligibility and Response: Immune Checkpoint Inhibitors. J Clin Oncol. 2024;42(16_suppl):e14613.
- Chennapragada SS, Ramadas P. Bispecific antibody toxicity. StatPearls. Last update April 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK603709/
- Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Bsel). 2023;15:713.
- D’Arienzo A, Verrazzo A, Pagliuca M, et al. Toxicity profile of antibody-drug conjugates in breast cancer: Practical considerations. EClinicalMedicine. 2023;62:102113.
URLs accessed on April 9, 2025